COVID-19 vaccine effectiveness in adolescents aged 12–17 years and interim public health considerations for administration of a booster dose
Given the emerging evidence of waning immunity in adults following a two-dose schedule with authorised vaccines and following requests from some Member States, the purpose of this document is to review the evidence of COVID-19 vaccine effectiveness and duration of immunity following vaccination in adolescents aged 12-17 years, and to outline interim public health considerations for the potential use of a booster dose in this group.
Executive summary
Key messages
- The vaccination of adolescents was introduced during the summer of 2021, approximately six months after COVID-19 vaccines were introduced in EU/EEA countries. All EU/EEA countries now recommend COVID-19 vaccination of adolescents aged 12-17 years old, and of these, ten also recommend a booster dose for those under 18 years of age.
- To date, at the EU level, the administration of booster doses is currently exclusively authorised for individuals 18 years of age and older. The EMA Committee for Medicinal Products of Human Use (CHMP) is currently evaluating data on the use of booster doses in adolescents.
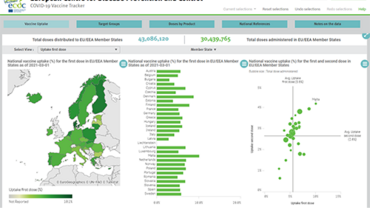
- As of week 4 (30 January 2022), the median uptake of the primary course of COVID-19 vaccine among adolescents aged 15-17 years old was 70.9% (range: 17.9-92.6%) and among 10-14 year-olds it was 34.8% (range: 3-63.8%) with broad heterogeneity across EU/EEA countries. More than half of adolescents aged 10 to 17 in the EU/EEA have not yet completed a primary course.
- SARS-CoV-2 notification rates of symptomatic disease in 12-17 year-olds have increased steadily since July 2021, largely mirroring the increased reporting rate observed in all age groups during the Delta and Omicron variant-dominated waves. However, a decrease in notification rates has been recently observed. The crude risk of hospitalisation, ICU admission and death remain very low for 12-17 year-olds.
- The studies available among adolescents mainly report vaccine effectiveness of the primary vaccination course against the Delta VOC and show a very high level of protection against infection, symptomatic disease, and severe disease.
- There is limited evidence available of waning of immunity following vaccination among adolescents. The available data suggest a waning of vaccine effectiveness against symptomatic infection five to six months following completion of the primary vaccination course, however no evidence of waning immunity against severe disease is currently available.
- There are currently limited data available on benefits and risks of a booster dose administered to adolescents who completed their primary vaccination course against COVID-19. Preliminary findings suggest an increase of vaccine effectiveness against documented SARS-CoV-2 infection in adolescents who received a booster compared to adolescents who have recently completed the primary vaccination course. However, no data are yet available on the duration of protection from a booster dose and on the additional effectiveness against severe disease of a booster dose in adolescents.
- Mathematical modelling suggests that the impact of administering a booster dose against COVID-19 to adolescents aged 12-17 years is a small reduction (1-3%) of the effective reproduction number (R(t)) in the whole population, varying according to the level of uptake of booster doses among adolescents.
- When considering the possibility of administering a booster dose to adolescents who completed the primary course, data on the benefit-risk of a booster dose in this age group should be carefully reviewed as they become available. Additionally, consideration should be given to the epidemiological situation, the national priorities and objectives of the COVID-19 vaccination campaign, the status of the rollout of the COVID-19 vaccine and of additional doses in priority groups and in the general population, as well as vaccine equity and supply.
- At this stage, priority should still be given to completion of the primary series in the eligible population and to administering booster doses to priority groups, before considering giving booster doses to adolescents aged 12-17 years with no underlying conditions.